ADORED-Allergic Disease Onset Prevention Study

Purpose:
Early administration of beneficial bacteria that promote immune tolerance represents a novel approach for the prevention and treatment of allergic diseases, such as atopic dermatitis and asthma. STMC-103H is a live biotherapeutic product (LBP) containing a consortium of intestinal bacteria that may impact the incidence of allergic sensitization and disease in neonates and infants at risk for developing allergy and asthma. STMC-103H is an oral capsule containing powder which will be mixed with a small amount of breastmilk, the participant’s formula, or a milk product. Siolta Therapeutics is testing this investigational drug for the prevention of allergic diseases, including atopic dermatitis (eczema), food allergy, asthma, and allergic rhinitis (hay fever).


The Study

About Clinical Trials
Diabetes
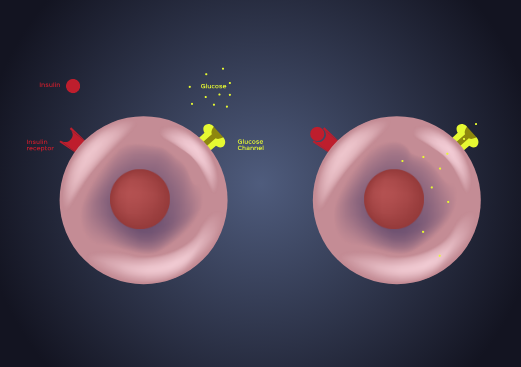
What is Diabetes?
What causes Diabetes?
How is Diabetes treated?
More information about Diabetes
Call 1800-9860-568 now to find out if you are eligible.
The Study
Who is eligibile?
What is the purpose of the study?
What is the study medicine?
About Clinical Trials
What is a clinical trial?
What are the phases of a clinical trial?
Why should I join a clinical research study?
What questions should I ask if I am thinking about a clinical research study?
What to expect if you participate?
Check your eligibility
The prescreener preview is complete. You may now close the preview.
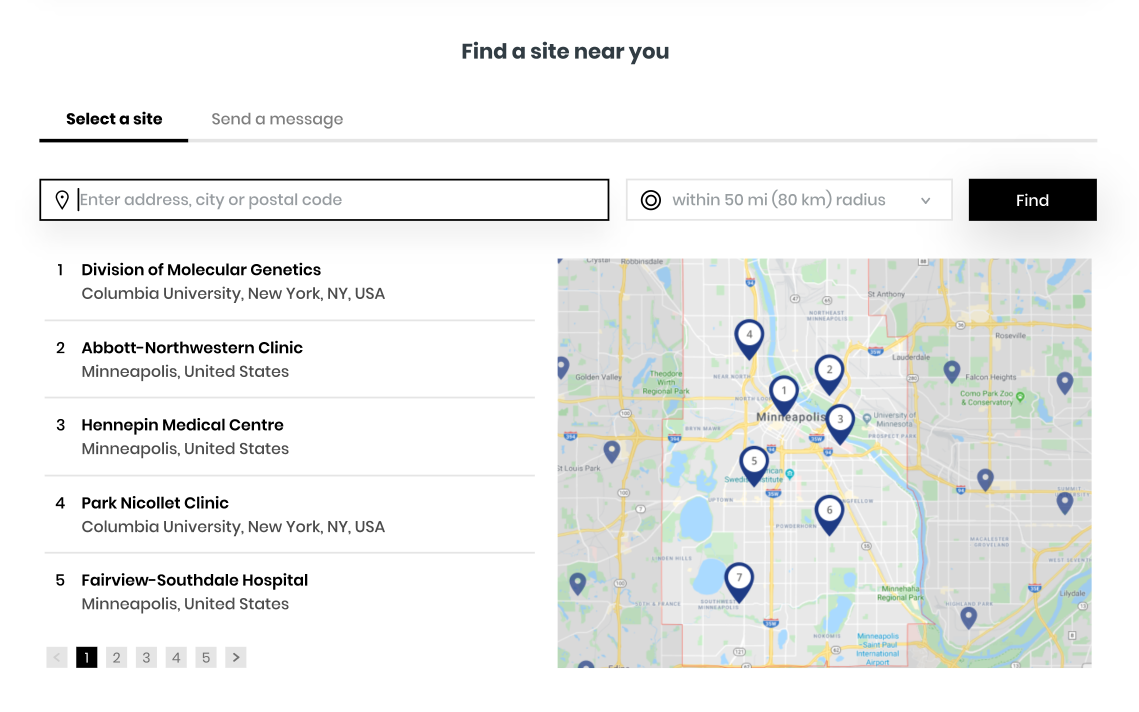
Find a site near you